Service Overview
Technical background
The process of linking monosaccharides (such as glucose, galactose) or polysaccharides to peptides through chemical bonds is called polypeptide glycosylation modification, and the peptides obtained through glycosylation modification are called Glycopeptides.
Glycopeptides often have important effects on the function of membrane proteins, and mediate specific biological functions, such as cell protection, stability, organization and barrier. It can be used as the specific ligand of exogenous receptors, and some sugar links can be used as the specific receptors of various viruses, bacteria and parasites. It can be used as a specific ligand of endogenous receptors and participate in mediating clearance, turnover and intracellular travel.
Technical principle
Artificial glycosylation of peptides mainly uses the hemiacetal hydroxyl group of sugar to form an α-amino group at the N end of the peptide, and the amide group of asparagine to form an n-glycosylation modification, or uses the hemiacetal hydroxyl group of sugar to form a -O-glucoside bond with the hydroxyl group of serine, threonine, hydroxylysine and hydroxyproline in the peptide.
1. N-terminal alpha-amino artificial glycosylation modification:
N-terminal α-amino artificial glycosylation modification commonly used sugars are glucose, fructose, glucosamine, galactose, galactosamine, etc.
We usually bind the sugar to the polypeptide by Schiff base reaction, where the aldehyde group of the sugar forms a CN double bond (-RC=N-) with the α-amino group of the polypeptide, but it should be noted that the peptide of the glycopeptide obtained by this method is an open-loop compound rather than a closed-loop compound.
2. O-glycoside linkage glycosylation modification
O-glycosidic bond glycosylation is mainly achieved by unit construction, that is, the acetylated protected sugar is first reacted with the hydroxyl group of serine, threonine, hydroxylysine and hydroxyproline to form a building unit, and the α-amino group of amino acids is protected by Fmoc, which is introduced at the desired location during the synthesis of peptides. After years of research and development, glucose, galactose, N-acetylglucosamine, GLC-βGLC, Man, sugar mimics, ribose and other building blocks connected with asparagine, serine, threonine, hydroxylysine and hydroxyproline have been successfully developed.
MOTIE Technical Advantages and Successful Cases
Service features
Each step of the glycopeptide synthesis process can be precisely controlled, including the difficulty of Ac removal, the selection of each glycosylation site and the degree of modification, which can ensure the quality of the product.
Success stories
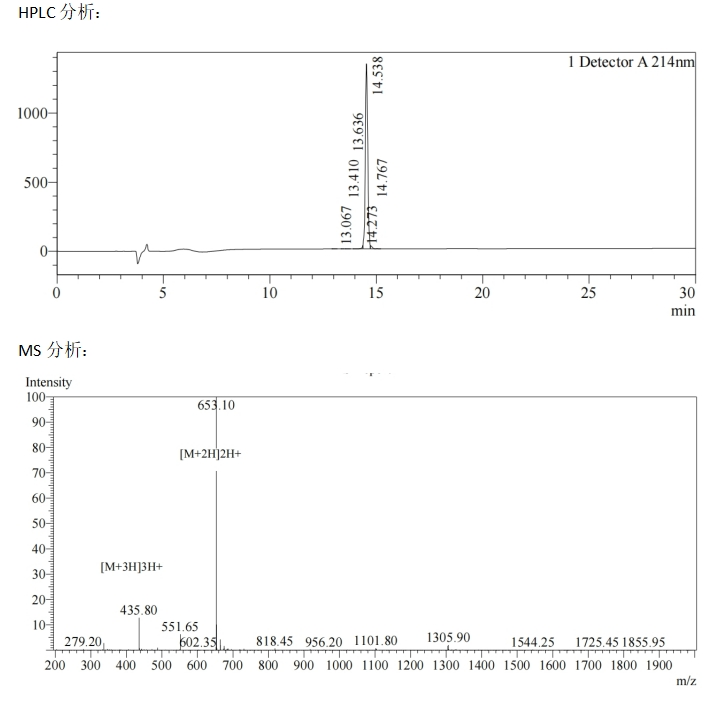
At present, MOTIF has successfully synthesized the sequence V**SS(O-GlcNAc)***RSP (Figure below).